All Controlled Drug Prescriptions Must Have Which of the Following
Controlled substance prescriptions have specific requirements. Further the Provider must complete a drug regimen review on all prescriptions filled as a result of the auto-refill program in accordance with 22 Tex.

Controlled Drug Prescribing Osce Guide Geeky Medics
PDMPs can provide health authorities timely information about prescribing and patient behaviors that contribute to the epidemic and facilitate a nimble and targeted response.

. Subscription Superscription Signa. Controlled drugs within Schedule 1 have little or no therapeutic value are addictive. That being said the pharmacist must ensure that the controlled.
1 There are no federal quantity limits on Schedule II prescriptions. This law requires that all written prescriptions issued by licensed prescribers in California be issued as electronic transmission prescriptions also known as e-prescriptions by Jan. Effective October 1 2007 prescriptions in written and non-electronic form that are not executed on a tamper-resistant pad as required by section 1903i23 of the Social.
Both have the potential to be abused or misused. MUST have all of the following. Patient name and address.
2 In addition there is no federal time limit on when a Schedule II prescription must be filled after being signed by a prescriber. Which of the following controlled substance schedule drugs can be obtained without a prescription. The signature of the authorised person witnessing the destruction.
The law details a penalty for noncompliance. The prescriber may need to contact the EHR or application provider to determine how to obtain these credentials. Controlled substances include both prescription drugs and illicit drugs with no recognized medical value.
See Arkansas E-Prescribing for further details. Controlled Substance Act USF-NF. A prescription drug monitoring program PDMP is an electronic database that tracks controlled substance prescriptions in a state.
Patients Full Name 3. The list shows each drugs respective classifications under both the Misuse of Drugs Act 1971 and the Misuse of Drugs Regulations 2001. Schedule II prescriptions must be presented to the pharmacy in written form and signed by the prescriber.
A pharmacy that will be dispensing controlled drugs must have which one of the following forms on file with the DEA. B2 outpatient prescriptions must have the elements listed in 29134b7 which are the same elements required to dispense a prescription by a Class A Community Pharmacy. A machine-written prescription is acceptable but the prescribers signature must be handwritten.
Colorado Senate Bill 79 proposes that all controlled substances Schedule II-IV be electronically prescribed. Controlled substances are drugs or chemicals that have the potential to be addictive or habit-forming. Prescriptions for inpatients must be written on the Ashtons Order Form and the original copy must be sent to Ashtons to comply with legal.
5 The electronic prescription application must accept two-factor authentication that meets the requirements of 1311115 and require its use for signing controlled substance prescriptions and for approving data that set or change logical access controls related to reviewing and signing controlled substance prescriptions. Obtain credentials to electronically sign controlled substance prescriptions. To be valid in addition to the normal prescription requirements for Prescription Only Medicines as required by the Human Medicines Regulations 2012 prescriptions for Schedule 2 and 3 CDs must also contain the following as outlined in The Misuse of Drugs Regulations 2001.
Prescription labels must contain all of the following information except. Drugs in this class may be utilized for pain control as long as the provider deems the drug to be medically necessary and that the patient would benefit. The date of destruction.
Dosage Form Schedule II Prescriptions US. This law requires that all prescriptions for controlled substances Schedule II-VI be electronically prescribed. All controlled drugs are listed in 1 of 5 Schedules to the 2001 Regulations according to their therapeutic usefulness their potential for abuse diversion and the perceived need for control.
Contract must require the Provider to confirm with the Member that a refill or new prescription received directly from the physician should be delivered. The United States Drug Enforcement Agency DEA divides controlled substances into 5 categories called Schedules based upon substances potential for abuse and addictiveness and its usefulness in medicine. In addition all pharmacies pharmacists or other practitioners authorized to dispense or furnish a medication must have the ability to receive.
The name strength and form of the controlled drug. While Schedule I drug use is illegal prescription drugs found in Schedules II-V are also commonly abused and misused and their misuse is a challenging problem that has increased over the. Prescriptions for Controlled Drugs that are subject to prescription requirements all preparations in Schedules 2 and 3 must be indelible must be signed by the prescriber include the date on which they were signed and specify the prescribers address must be within the UK.
Examples of Schedule IV substances include. CSA 7458 9663 4000 4000 Controlled Substances - Alphabetical Order - DEA SUBSTANCE NUMBER SCH NARC OTHER NAMES 1-4-Fluorobenzyl-1H-indol-3-yl2233- 7014 I N FUB-144. MaineCare does not reimburse for the following drugs or products as drugs.
A prescription must contain all of the following information before a pharmacy can consider filling the prescription. Professional License Controlled Substance License Drug Enforcement Administration DEA Registration Drug Control License Exempt for samples or veterinarians A MAPS Claim Form must be completed for controlled substance that is dispensed includieach ng refills and. Have a certification report for the Electronic Health Records EHR or electronic prescription application to verify compliance with the DEA rule.
All prescriptions for controlled substances must include the following. A All prescriptions for controlled substances shall be dated as of and signed on the day when issued and shall bear the full name and address of the patient the drug name strength dosage form quantity prescribed directions for use and the name address and registration number of the practitioner. It is a legal requirement under the Misuse of Drugs Act for prescriptions for controlled drugs CDs to contain certain details and it is not possible to dispense CDs if the prescription is not completed properly.
There is no requirement in the 2001 regulations that the disposal of date-expired medicines in Schedules 3 4 and 5 to be witnessed or recorded. Drug Enforcement Administration Diversion Control Division. A six digit number on a prescription drug card that is used for routing and identification to process a prescription claim.
Alprazolam carisoprodol clonazepam clorazepate diazepam lorazepam midazolam temazepam tramadol and triazolam.
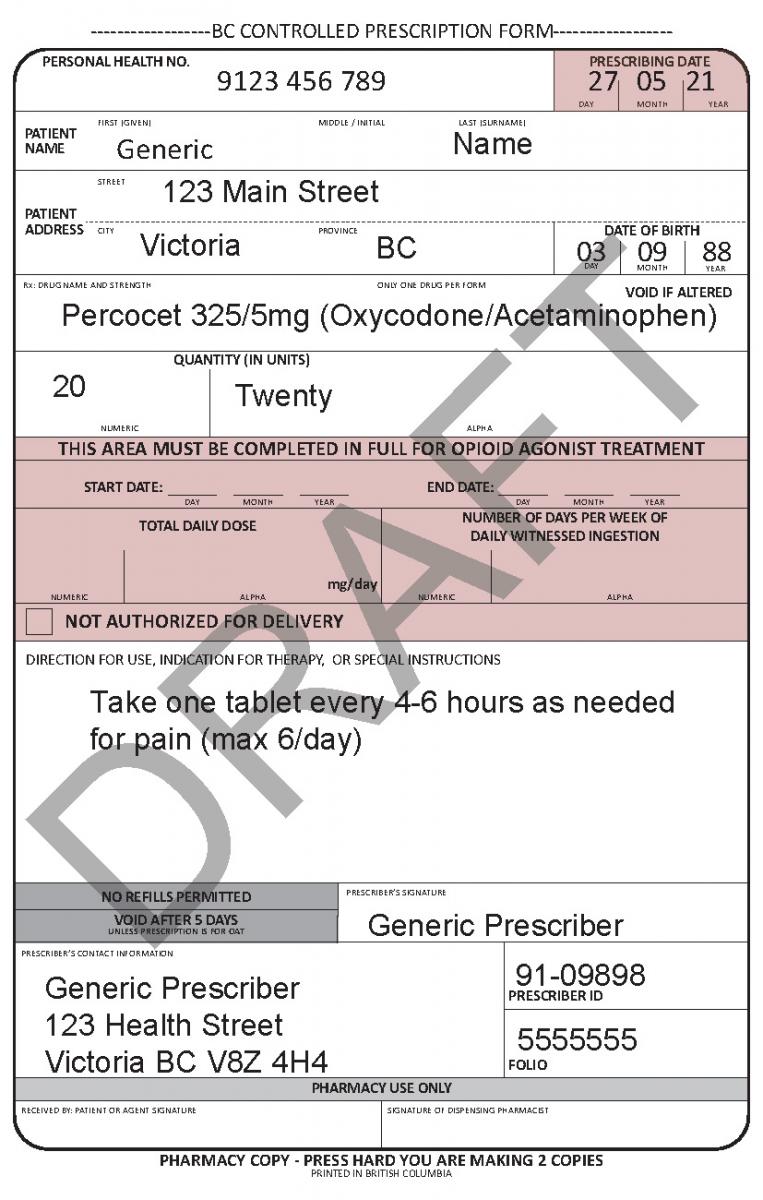
Controlled Prescription Program College Of Pharmacists Of British Columbia

The Federal Controlled Substances Act A Primer For Providers
0 Response to "All Controlled Drug Prescriptions Must Have Which of the Following"
Post a Comment